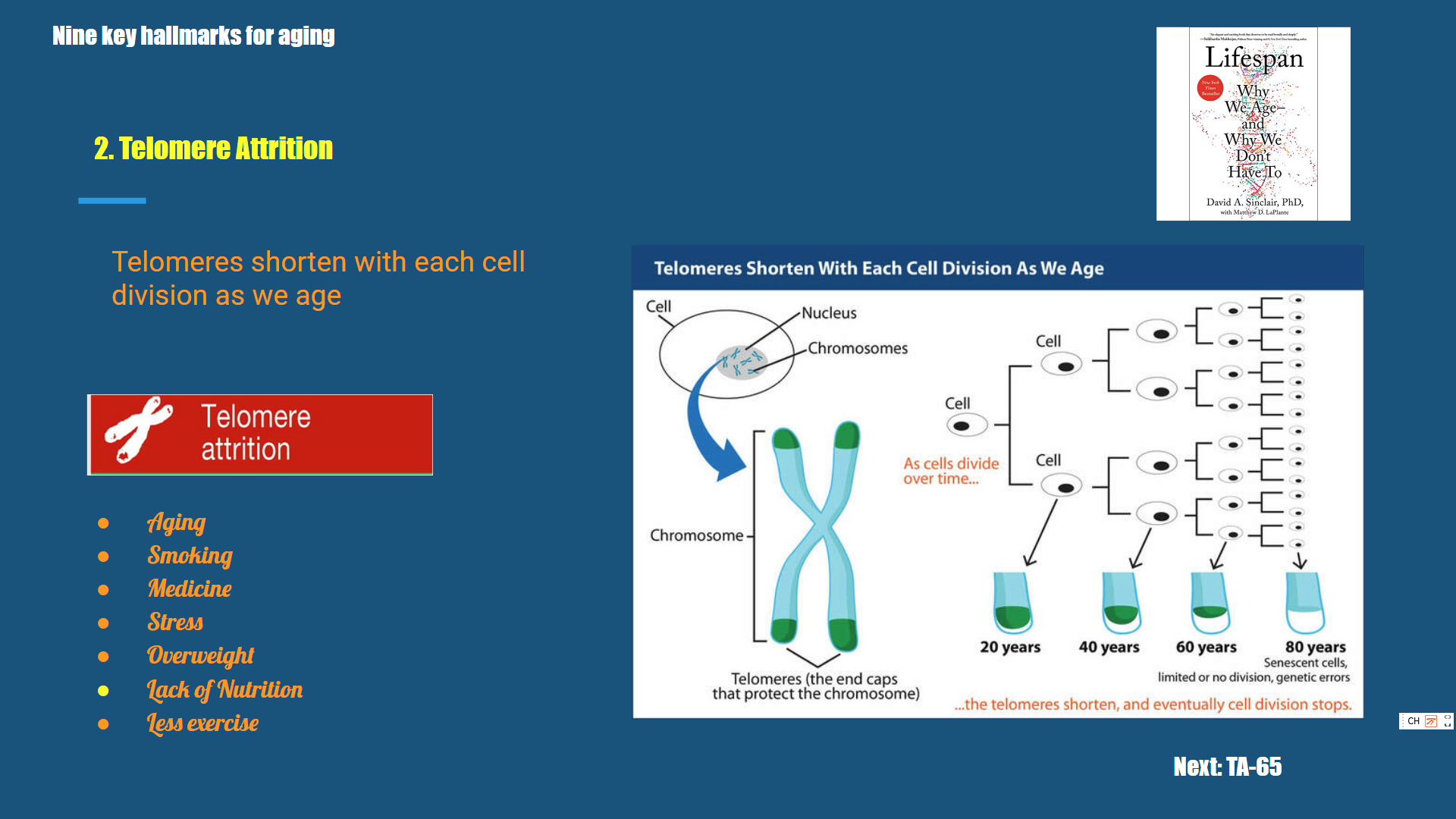
Telomeres are repetitive nucleotide sequences that protect the ends of chromosomes and maintain genomic stability (Fig. 5). Telomere dysfunction and genomic instability appear to be of critical importance for ageing at a cellular level. Age-related diseases and premature ageing syndromes are frequently associated with telomere shortening.

In 1961, Hayflick and Moorhead noted that human diploid cells lines could be cultured for only a limited number of passages. This gave rise the telomerase theory of ageing and the Hayflick limit . The basis for this theory is the observation that most human somatic cells do not express telomerase, an enzyme capable of extending the telomere ends of human DNA. Human DNA polymerase is incapable of replicating the telomere ends of DNA fully. Thus, each cell division leads to progressive shortening of the DNA strands; eventually, in theory, this progresses into the coding parts of the DNA (Fig. 5). To avoid this, the cells enter replicative senescence after a certain number of divisions and associated telomere shortening, known as the Hayflick limit.
In people aged over 60 years, short telomere length is associated with earlier death from age-related diseases. Telomerase overexpression can inhibit ageing, but at the expense of increased tumorigenesis. A study of long-lived families found that telomere length was shorter with age in men; importantly, it appeared that telomere length was highly heritable. Finally, telomere shortening is associated with a number of age-related diseases such as osteoarthritis, atherosclerosis, coronary heart disease and atrial fibrillation.


From : https://bjssjournals.onlinelibrary.wiley.com/doi/10.1002/bjs.10053