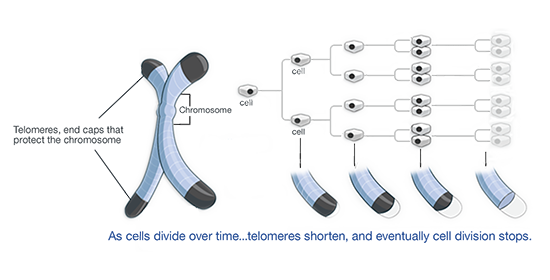
Telomere Shortening and Telomerase.
Content
- What are telomeres?
- Telomeres shorten with each cell division as we age
- Shorter telomeres. So what?
- Rates of telomere shortening vary from person to person: genetic and lifestyle factors. Controversial data
- Telomerase. A solution to the telomere shortening?
- Maintaining or improving telomere length. Practical Recommendations
- Summary
- References and literature
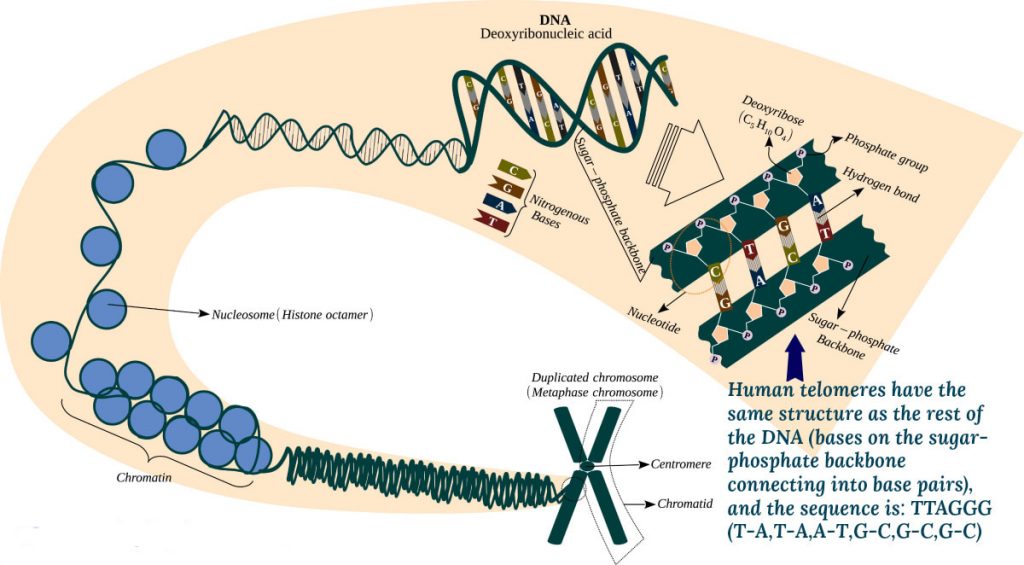
What are Telomeres?
In humans, the repeated sequence of nitrogenous bases in telomeres is TTAGGG.In plain words, telomeres are like protective caps at the end of the chromosome tails. They protect data-carrying portions, genetic information, of the chromosomal DNA.
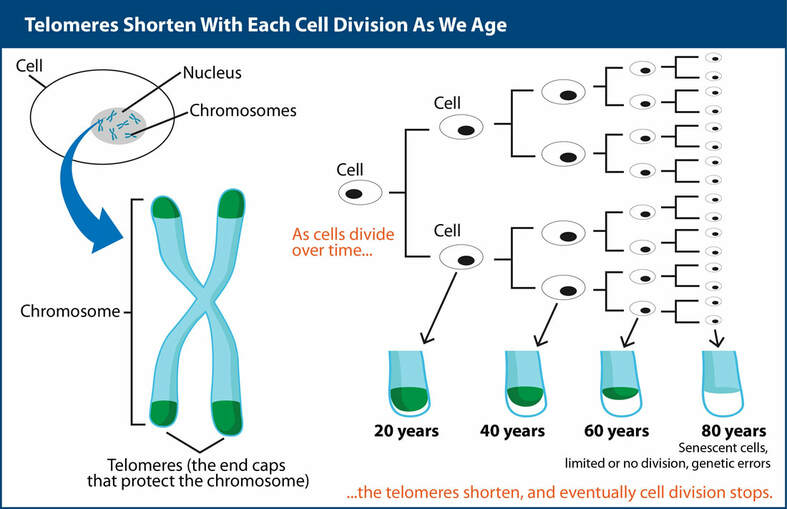
Telomeres shorten with each cell division as we age
Throughout our lives, cells replicate. Cell replication includes replication of the chromosomes.
The typical schematic depiction of a chromosome as an X resembles the way a chromosomes actually looks like precisely when the DNA has been just duplicated during the cell division. When a cell is not replicating its chromosomes, a chromosome looks like a blob of yarn.
In the absence of a compensatory mechanism, with each DNA duplication during cell division, telomeres progressively shorten losing approximately 50 to 200 base pairs per cell division [Harley, 1990; Huffman, 2000; Martens, 2000; Wong, 2003]. The reason for that is what is called ‘end replication’ problem; the regular DNA polymerase enzyme that copies our chromosomal DNA during the cell replication cannot fully synthesize the chromosomal telomere ending on the what is called ‘lagging strand’.
Telomere length shortens in stem cells at slower rates than at those of somatic (non stem) cells, but it still does [Flores, 2008] .
Shorter telomeres. So what?
After about 50 to 75 cell divisions in humans (Hayflick limit) a telomere becomes so critically short that a cell can no longer replicate and becomes old; the term is “senescent cell” (there are other reasons as well why a cell may become senescent).
Thus, the telomere shortening that accompanies DNA copying during each cell division, limits the number of times a cell can divide. It acts as a biological clock of aging. Telomere length is one of the bio-markers of aging and biological age.The following are the known implications of the progressive telomere shortening –
- A cell that approaches the end of its reproductive life may sustain, due to shortened telomeres, a genetic damage that is associated with malignant neoplasms (cancerous tumors) [Wentzensen, 2011; Willeit, 2010];
- Shorter telomeres may lead to activation of the genes related to the old age disease and/or silencing of some of the genes. As telomeres become shorter, gene expression is affected [Benetos, 2001; Mondoux, 2005: Telomere position effect: silencing near the end]. One study [Stadler, 2013] showed that as the telomeres become shorter, normally silenced genes DUX4 and FRG2 may become active, thus leading to facioscapulohumeral muscular dystrophy (FSHD). Given that FRG2 is located at about 100 kilobases away from telomeres, the above finding suggests that due to shrinking telomeres, many genes related to the old age disease may become active, not only the ones located close to telomeres;
- Accelerated telomere shortening and shorter telomeres (typically, leukocyte telomere length) are associated with an increase in morbidity and mortality from a range of age-related diseases, including cardiovascular disease, chronic obstructive pulmonary disease, degenerative disc disease, Alzheimer’s disease, infectious diseases, osteoarthritis, rheumatoid arthritis, osteoporosis, macular degeneration, and other degenerative diseases [Honig, 2012; Ameh, 2017; Cohen, 2013; Bhattacharyya, 2017; Yeh, 2016; Fragkiadaki, 2020; Manoy, 2020; Savage, 2018];
- Several studies have shown that in particular the length of telomeres in leukocytes is an accurate risk factor predictor for the old-age disease [Aviv A, 2006; Benetos, 2001; Cawthon, 2003; Farzaneh-Far, 2008; Thomas, 2008].
- A senescent cell with critically short telomeres eventually acquires what is termed senescence-associated secretory phenotype (SASP). It means that a cells starts secreting toxic molecules into the outside environment. [Watanabe, 2017]. Those are so-called ‘zombie cells’. In a more scientific language, cells with SASP have upregulated genes that encode certain secreted proteins, such as inflammatory cytokines, chemokines, extracellular matrix remodeling factors, and growth factors. That senescent gene expression and excretion may lead to tissue degeneration, chronic inflammation, and tumor promotion. This process occurs in conjunction with at least several more aging related mechanisms and processes: malfunctioning of the cellular apoptosis through mitochondrial malfunction through, among other things, decrease in the selective macroautophagy and also an exhaustion of the stem cells.
The bottom line is that to stay young, we want longer telomeres.
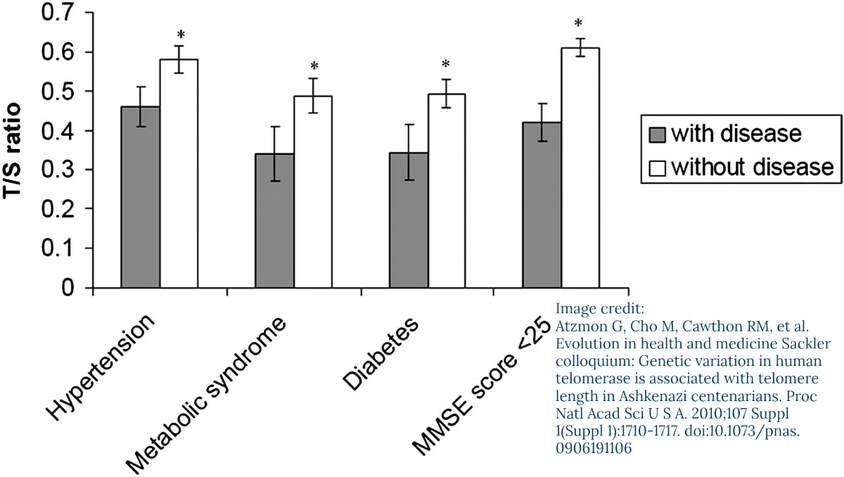
Rates of Telomere Shortening Vary from Person to Person
Telomere length maintenance may be to some extent heritable through variants in the genes encoding for hTERT and hTERC: the telomerase itself and the RNA template component (the next section covers telomerase).
This study [Atzmon, 2010] found an association (not necessarily causation) between certain variants in those two genes and exceptional longevity that runs in families. The study states, “our study demonstrates that centenarians may harbor individually rare but collectively more common genetic variations in genes involved in the telomere maintenance pathway”.Lifestyle Factors
Certain lifestyle factors too are associated with shorter (or longer) telomere length: obesity, stress, and receiving sufficient nutrients.
- Obesity
The 2009 study [Kim, 2009] looked at 647 women ages 35-74.
“When current BMI and BMI at ages 30–39 were considered together, the most marked decrease in telomere length was found for women who had overweight or obese BMI at both time points.” Weight cycling (loosing and gaining weight) was also inversely associated with the telomere length.
(the graphs included in the published paper show an approximately 500 base pair difference in the telomere length between women with the BMI <22.5 and BMI >25).
- Chronic stress may have an impact
This small observational study [Epel, 2004] compared telomere lengths of 39 chronically stressed mothers caring for chronically ill children for 1 to 12 years to the telomere length of 19 “control” mothers of healthy children. The study concluded that, “Women with the highest levels of perceived stress have telomeres shorter on average by the equivalent of at least one decade of additional aging compared to low stress women.” (550 base pair difference)
This 2019 study [Wang, 2017] analyzed leukocyte telomere length in 181 persons with anxiety, depression, or chronic stress with 320 individuals without those issues and concluded that, “Our findings confirm that telomere length, as compared with healthy controls, is shortened in patients with depression, anxiety and stress and adjustment disorders.”
Regarding this study, it is not possible to conclude whether it is shorter telomeres that had an impact on the psychological health or vice versa.
- Nutrients
A very small Greek study [Tsoukalas, 2019] found an association between telomere length and supplementation with a multi-vitamin, omega-3, probiotics and other nutrients. 16 people were in the Nutritional supplements group and 31 in the control group. After 6 to 12 months of supplementation, participants in the experimental group had, on average, approximately 900 base pairs longer leukocyte telomere length than did the participants in the control group.
If the results are correct and accurate and can be replicated, 900 base pairs is a significant difference, given that annual telomere attrition in adults is 31 – 65 bp/year.
- Short term stress may have minimal or no impact
This 2016 meta-analysis looked at the impact of the short-term stress on the telomere length and concluded that, “Our analysis finds a very small, statistically significant relationship between increased Psychological Stress (as measured over the past month) and decreased Telomere Length that may reflect publication bias. The association may be stronger with known major stressors and is similar in magnitude to that noted between obesity and TL. All included studies used single measures of short-term stress; the literature suggests long-term chronic stress may have a larger cumulative effect.”
- Physical Activity
Surprisingly, to date studies on the impact of physical activity on the telomere length are not fully conclusive. This 2015 meta analysis [Mundstock, 2015] stated, ‘Thirty-seven original studies were included in this systematic review, including 41,230 participants. Twenty articles did not find statistically significant association, whereas 15 described a positive association. Two papers found an inverted “U” correlation. There is a tendency toward demonstrating an effect of exercise on telomere length.’
Controversial data on the lifestyle factors impact on telomere length
- A choice of a particular diet
What is the best diet for the telomere length maintenance?
This 2017 systematic review and meta-analysis (the highest quality of the scientific evidence) [Perez, 2017] reviewed “RCTs that evaluated the effects on telomere length of the following diets: calorie restriction, high-fat diet, Mediterranean diet, micronutrient supplementation, or combinations of different interventions.”
The study concluded that, “The available evidence suggests that there is no effect of diet on telomere length, but the strong heterogeneity in the type and duration of dietary interventions does not allow any final statement on the absence of an effect of diet on telomere length.”
- Smoking
Even more curious is the conclusion of this 2019 meta-analysis of longitudinal cohorts [Bateson, 2019]:
“the difference in LTL (leukocyte telomere length) between smokers and non-smokers is extremely unlikely to be explained by a linear, causal effect of smoking. Selective adoption, whereby individuals with short telomeres are more likely to start smoking, needs to be considered as a more plausible explanation for the observed pattern of telomere dynamics.”
The study conclusions obviously do not suggest to take up smoking or to switch to an unhealthy diet.
Telomerase. A solution to the telomere shortening?
Telomerase is an RNA-dependent DNA polymerase and a reverse transcriptase, that consisted of two parts, the telomerase reverse transcriptase as such (TERT) and the RNA component (TERC). To reword, telomerase is an enzyme complex that takes the RNA template and builds the telomeric ends of the DNA.
Our genes encode for telomerase, and telomerase is very active during the embryonic development. It is also highly expressed in proliferating stem-like cells, specific germline cells, and many cancers. Apart from that, telomerase in humans becomes inactive or expressed at very low levels that are not sufficient to maintain telomere length.
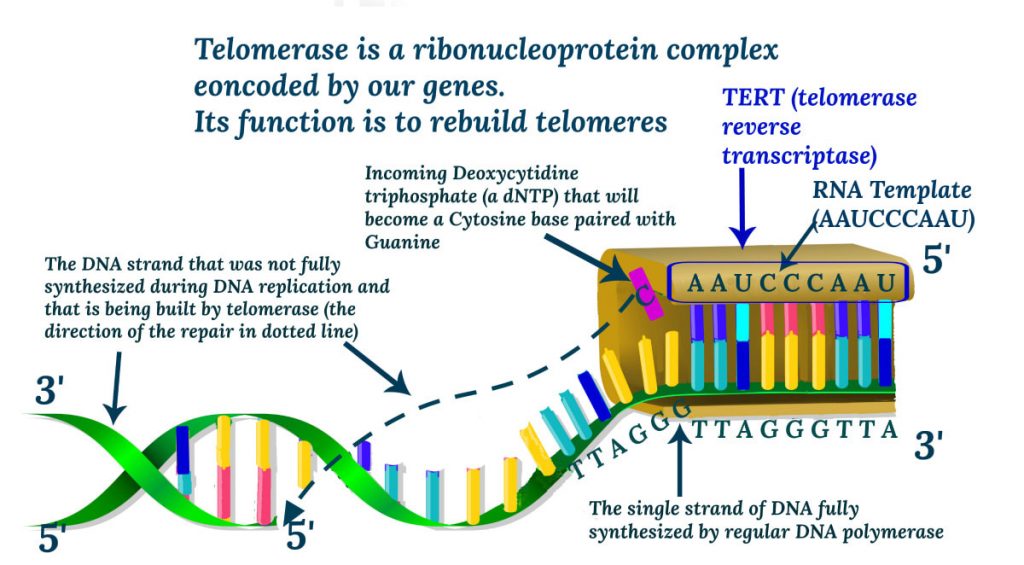
For this reason, activation of telomerase became one of the top targets of the biotechnology, gene therapy. With that approach, the recipient is treated with additional genes that will be transcribed and translated to telomerase.A number of companies and scientists are also searching for a drug-like compound that would activate telomerase that our own genes already encode.
Maintaining or improving telomere length. Practical recommendation
As discussed above, lifestyle interventions that may assist with maintaining or improving telomere length are —
- Maintain healthy weight, BMI under 25;
- Consume sufficient nutrients such as vitamins, minerals, omega-3 fatty acids, possibly by supplementing your diet with a combination of high quality (proper form and dose, from a reliable manufacturer) vitamins, minerals, and omega-3;
- Deal with stress: either minimize exposure to stressful stimuli, or learn tools how to not get stressed in response to the stimuli, or learn tools how to effectively process stress and emotions after you experience them;
- Consider limiting the food consumption window to 8 or at least 10 hours a day (no food or calories in the remaining 16 or 14 hours);
- Stay physically active.
Supplements
In this section I will cover supplements only briefly.
There are currently no compounds proven to significantly extend telomeres in humans, whether by telomerase activation or via an alternative route. There are, however, compounds and formulations that may have some limited effectiveness.
1. As covered above — consider taking a high quality combination (proper form and dose) of vitamins, minerals, omega-3, and other vitamin-like compounds,
2. TA-65, or
3. The “generic version” of TA-65 — cycloastragenol, an extract from Astragalus Membranaceus,
4. DefyTime by Sierra Sciences,
5. A concentrated extract from Gotu Kola (Centella asiatica) [Tsoukalakas, 2019] was found to be the most potent telomerase activator compared to TA-65, astragalus extract, and other compounds.
Keep in mind that,
1) it has only been tested in vitro, in [human blood] cell culture, not even on mice. A lot happens in a body between when a compound is consumed and when it (or its metabolite) reaches cells, therefore when digested, the Gotu Kola extract may not actually have any telomerase activation effect,
2) the extract used in the study was a Centella asiatica extract which consisted of >95% high-purity triterpenes. The highest concentration I was able to find contains 35-45% triterpenes.
I am somewhat optimistic about the Gotu Kola extract though because for centuries the plant has been used as a brain tonic and tissue healing medicinal plant in the traditional Eastern medicine.
6. Supplementation with an NAD+ precursor, which may increase NAD+ levels that may activate sirtuins that may stabilize telomere length. Theoretically that route works. In reality it may be a long shot, however, taking an NAD+ precursor after 35 is a good idea even apart from its possible impact on the telomere length.
Summary
- Telomeres are protective caps at the end of the chromosomal DNA;
- Telomeres shorten as we age during the natural process of cell division;
- Chronic stress, obesity, smoking, poor nutrition, and lower socio-economic status are associated with shorter telomere length;
- Telomere length is one of the well-established biomarkers of aging;
- To stay young, we need longer telomeres;
- Shorter telomeres are associated with many age-related diseases and a shorter life-span;
- Telomere shortening may be to some extent impacted through life-style changes. The most promising are: maintaining BMI under 22.5, receiving all the nutrients, stress management (minimize exposure to the stress factors or learn to not perceive them as stress or learn to manage the perceived stress);
- Some compounds may assist with maintaining telomere length;
- Telomere shortening and telomerase activation are among the top therapeutical targets against aging.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3732483/
Ameh O. I., Okpechi I. G., Dandara C., Kengne A. P. Association between telomere length, chronic kidney disease, and renal traits: a systematic review. OMICS. 2017;21(3):143–155. doi: 10.1089/omi.2016.0180. https://pubmed.ncbi.nlm.nih.gov/28253088/Atzmon G, Cho M, Cawthon RM, et al. Evolution in health and medicine Sackler colloquium: Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc Natl Acad Sci U S A. 2010;107 Suppl 1(Suppl 1):1710-1717. doi:10.1073/pnas.0906191106
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2868292/
Aviv A: Telomeres and human somatic fitness. J Gerontol A Biol Sci Med Sci. 2006 Aug; 61(8): 871-3 https://www.ncbi.nlm.nih.gov/pubmed/16912107
Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B., Kirkland J.L., van Deursen J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3468323/
Baker D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J., Saltness R.A., Jeganathan K.B., Verzosa G.C., Pezeshki A., Khazaie K., Miller J.D., van Deursen J.M. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4845101/
Bateson M, Aviv A, Bendix L, et al. Smoking does not accelerate leucocyte telomere attrition: a meta-analysis of 18 longitudinal cohorts. R Soc Open Sci. 2019;6(6):190420. Published 2019 Jun 5. doi:10.1098/rsos.190420
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6599800/
Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A: Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001 Feb; 37(2 part 2): 381-5. https://www.ncbi.nlm.nih.gov/pubmed/11230304
Bhattacharyya J, Mihara K, Bhattacharjee D, Mukherjee M. Telomere length as a potential biomarker of coronary artery disease. Indian J Med Res. 2017;145(6):730–737. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5674542/
Blackburn EH: Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573 https://www.ncbi.nlm.nih.gov/pubmed/1708110
Blackburn EH: Switching and signaling at the telomere. Cell. 2001 Sep 21;106(6):661–673. https://www.cell.com/cell/fulltext/S0092-8674(01)00492-5
Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA:Association between telomere length in blood and mortality in people aged 60 years or older. Lancet.2003 Feb;361:393–395. https://www.ncbi.nlm.nih.gov/pubmed/12573379
Cohen S, Janicki-Deverts D, Turner RB, et al. . Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA 2013; 309: 699–705. doi:10.1001/jama.2013.613 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3786437/
Dagnall CL, Hicks B, Teshome K et al. Effect of pre-analytic variables on the reproducibility of qPCR relative telomere length measurement. PLOS ONE. September 8, 2017 doi:10.1371/journal.pone.0184098. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5590866/
Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312-17315. doi:10.1073/pnas.0407162101
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC534658/
Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA: Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008 Jul;28(7):1379-1384 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2675880/
Flores I, Canela A, Vera E, Tejera A, Cotsarelis G, Blasco MA. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 2008;22(5):654-667. doi:10.1101/gad.451008
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2259034/
Fragkiadaki P, Nikitovic D, Kalliantasi K, Sarandi E, Thanasoula M, Stivaktakis PD, Nepka C, Spandidos DA, Tosounidis T, Tsatsakis A. Telomere length and telomerase activity in osteoporosis and osteoarthritis. Exp Ther Med. 2020;19:1626–1632. doi: 10.3892/etm.2019.8370. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7027092/
Harley CB, Futcher AB, Greider CW: Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31; 345(6274):458-60. https://www.ncbi.nlm.nih.gov/pubmed/2342578/
Hayflick L.: The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965 Mar; 37:614–636. https://www.ncbi.nlm.nih.gov/pubmed/14315085
Honig L. S., Kang M. S., Schupf N., Lee J. H., Mayeux R. (2012). Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch. Neurol. 69, 1332–1339. 10.1001/archneurol.2012.1541 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3622729/
Huffman KE, Levene SD, Tesmer VM, Shay JW, Wright WE: Telomere shortening is proportional to the size of the G-rich telomeric 3′-overhang. J Biol Chem.2000 Jun 30; 275(26):19719-22. http://www.jbc.org/content/275/26/19719.long
Kim S, Parks CG, DeRoo LA, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009;18(3):816-820. doi:10.1158/1055-9965.EPI-08-0935
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2805851/
Lim S, Yahya Z, Zeegers D, Moe T, Kyaw EEP, Yeo GSH, Hande MP, Tan EC: Distribution of telomere length in the cord blood of Chinese newborns. Br J of Medicine & Medical Research. 2013 Oct – Dec; 3(4): 1004-1014.
http://www.sciencedomain.org/abstract/1101
Manoy P, Yuktanandana P, Tanavalee A, Tanpowpong T, Ittipanichpong T, Honsawek S. Telomere shortening is associated with poor physical performance in knee osteoarthritis. Biomed Rep. 2020;13(4):27. doi:10.3892/br.2020.1334 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7403814/
Martens UM, Chavez EA, Poon SS, Schmoor C, Lansdorp PM: Accumulation of short telomeres in human fibroblasts prior to replicative senescence. Exp Cell Res. 2000 Apr 10; 256(1):291-9. https://www.ncbi.nlm.nih.gov/pubmed/10739676/
Mondoux, M. A., and V. A. Zakian, 2005. Telomere position effect: silencing near the end, pp. 261–316 in Telomeres, Ed. 2, edited by T. de Lange, V. Lundblad and E. H. Blackburn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Mundstock E, Zatti H, Louzada FM, et al. Effects of physical activity in telomere length: Systematic review and meta-analysis. Ageing Res Rev. 2015;22:72-80. doi:10.1016/j.arr.2015.02.004
https://pubmed.ncbi.nlm.nih.gov/25956165/
Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A:Telomere Length in the Newborn. Pediatric Research.2002 Sep; 52, 377–381.
https://www.nature.com/articles/pr2002193
Olovnikov AM: A theory of marginotomy: The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. Laboratory of Chemistry and Synthesis of Antibodies, Gamaleya Institute for Epidemiology and Microbiology, Moscow, USSR. 1972/J Theor Biol. 1973 Oct; 41(1):181-189 https://www.ncbi.nlm.nih.gov/pubmed/4754905
Pérez LM, Amaral MA, Mundstock E, et al. Effects of Diet on Telomere Length: Systematic Review and Meta-Analysis. Public Health Genomics. 2017;20(5):286-292. doi:10.1159/000486586
https://pubmed.ncbi.nlm.nih.gov/29439273/
Savage SA. Beginning at the ends: telomeres and human disease. F1000Res. 2018;7:F1000 Faculty Rev-524. Published 2018 May 1. doi:10.12688/f1000research.14068.1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5931273/
Stadler G, Rahimov F, King OD, Chen JC, Robin JD, Wagner KR, Shay JW, Emerson CP Jr, Wright WE: Telomere position effect regulates DUX4 in human facioscapulohumeral muscular dystrophy. Nat Struct Mol Biol.2013 Jun;20(6):671-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3711615/
Thomas P, O’ Callaghan NJ, Fenech M: Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev.2008 Apr;129:183–190.https://www.ncbi.nlm.nih.gov/pubmed/18242664
Tsoukalas D, Fragkiadaki P, Docea AO, et al. Association of nutraceutical supplements with longer telomere length. Int J Mol Med. 2019;44(1):218-226. doi:10.3892/ijmm.2019.4191
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6559326/
Tsoukalas D, Fragkiadaki P, Docea AO, et al. Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives. Mol Med Rep. 2019;20(4):3701-3708. doi:10.3892/mmr.2019.10614
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6755196/
Victorelli S, Passos JF. Telomeres and cell senescence – size matters not. EBioMedicine. 2017;21:14–20. doi: 10.1016/j.ebiom.2017.03.027.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5514392/
Wang X, Sundquist K, Hedelius A, Palmér K, Memon AA, Sundquist J. Leukocyte telomere length and depression, anxiety and stress and adjustment disorders in primary health care patients. BMC Psychiatry. 2017;17(1):148. Published 2017 Apr 24. doi:10.1186/s12888-017-1308-0
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5404668/
Watanabe S, Kawamoto S, Ohtani N, Hara E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017;108:563–569. doi: 10.1111/cas.13184. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5406532/
Wentzensen IM, Mirabello L, Pfeiffer RM,Savage SA: The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011 Jun; 20(6):1238-50. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3111877/
Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstätter A, Kronenberg F, Kiechl S: Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010 Jul 7;304(1):69-75. https://www.ncbi.nlm.nih.gov/pubmed/20606151
Wong JM, Collins K. Telomere maintenance and disease. Lancet. 2003;362(9388):983-988. doi:10.1016/S0140-6736(03)14369-3
https://pubmed.ncbi.nlm.nih.gov/14511933/
Yeh JK, Wang CY. Telomeres and telomerase in cardiovascular diseases. Genes (Basel). 2016;710.3390/genes7090058 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5042389/
Yonekawa T, Thorburn A. Autophagy and cell death. Essays Biochem. 2013;55:105-17.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3894632/